Recent Publications

She, X., Moore, B.J., Roeder, B.M., Nune, G., Robinson, B.S., Lee, B., Shaw, S., Gong, H., Heck, C., Popli, G., Couture, D., Laxton, A., Marmarelis, V.Z., Liu, C., Deadwyler, S.A., Berger, T.W., Hampson, R.E., and Song, D. Distributed temporal coding of visual memory categories in human hippocampal neurons revealed by an interpretable decoding model. Advanced Science, 2025, DOI: 10.1002/advs.202502047.
The hippocampus is crucial for forming new episodic memories. While its role in encoding spatial and temporal information (where and when) is well understood, how it encodes objects (what) remains unclear due to the high dimensionality of object space. Rather than encoding each object separately, the hippocampus may encode object categories to reduce complexity. Here, an experimental-modeling approach to investigate how the hippocampus encodes visual memory categories in humans is developed. Spikes are recorded from hippocampal CA3 and CA1 neurons in 24 epilepsy patients performing a delayed match-to-sample task involving five image categories. An interpretable memory decoding model is employed to decode memory categories from hippocampal spiking activity and identify the spatio-temporal characteristics of hippocampal encoding. Using this model, the optimal temporal resolutions for decoding each visual memory category per neuron are estimated. Results indicate that visual memory categories can be decoded from hippocampal spike patterns, supporting the presence of category-specific coding. Hippocampal neuron ensembles encode memory categories in a distributed manner, akin to a population code, while individual neurons use a temporal code. Additionally, CA3 and CA1 neurons exhibit similar and redundant memory category information, likely due to strong and diffuse feedforward synaptic connections from CA3 to CA1 regions.
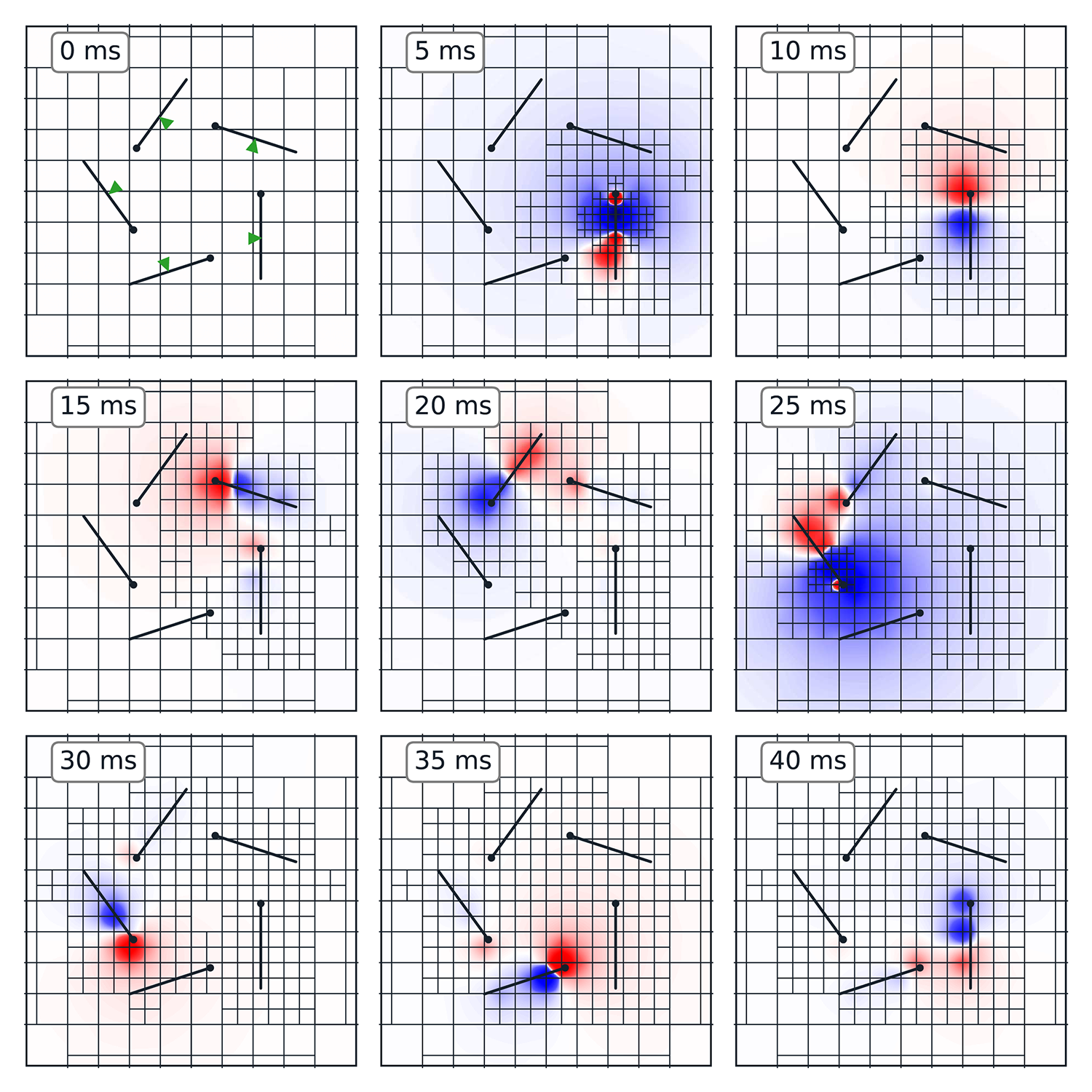
Girard, C., and Song, D. Adaptive octree meshes for simulation of extracellular electrophysiology. Journal of Neural Engineering, 2023, DOI: 10.1088/1741-2552/acfabf.
Objective. The interaction between neural tissues and artificial electrodes is crucial for understanding and advancing neuroscientific research and therapeutic applications. However, accurately modeling this space around the neurons rapidly increases the computational complexity of neural simulations. Approach. This study demonstrates a dynamically adaptive simulation method that greatly accelerates computation by adjusting spatial resolution of the simulation as needed. Use of an octree structure for the mesh, in combination with the admittance method for discretizing conductivity, provides both accurate approximation and ease of modification on-the-fly. Main results. In tests of both local field potential estimation and multi-electrode stimulation, dynamically adapted meshes achieve accuracy comparable to high-resolution static meshes in an order of magnitude less time. Significance. The proposed simulation pipeline improves model scalability, allowing greater detail with fewer computational resources. The implementation is available as an open-source Python module, providing flexibility and ease of reuse for the broader research community.
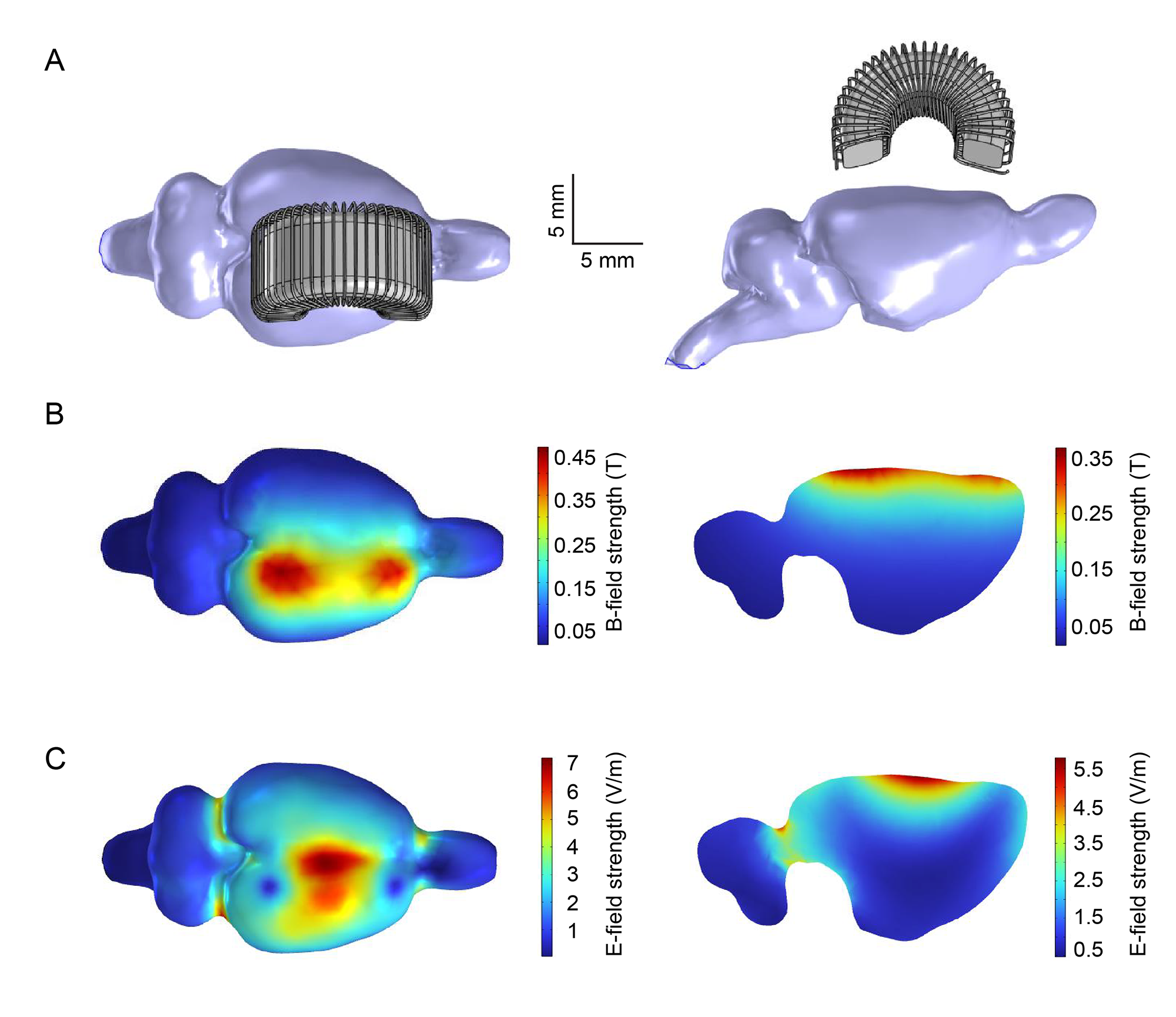
Jiang, W., Isenhart, R., Liu, C.Y., and Song, D. A C-shaped miniaturized coil for transcranial magnetic stimulation in rodents. Journal of Neural Engineering, 2023, DOI: 10.1088/1741-2552/acc097.
Objective. Transcranial magnetic stimulation (TMS) is a non-invasive technique widely used for neuromodulation. Animal models are essential for investigating the underlying mechanisms of TMS. However, the lack of miniaturized coils hinders the TMS studies in small animals, since most commercial coils are designed for humans and thus incapable of focal stimulation in small animals. Furthermore, it is difficult to perform electrophysiological recordings at the TMS focal point using conventional coils. Approach. We designed, fabricated, and tested a novel miniaturized TMS coil (4-by-7 mm) that consisted of a C-shaped iron powder core and insulated copper wires (30 turns). The resulting magnetic and electric fields were characterized with experimental measurements and finite element modeling. The efficacy of this coil in neuromodulation was validated with electrophysiological recordings of single-unit activities (SUAs), somatosensory evoked potentials (SSEPs), and motor evoked potentials (MEPs) in rats (n = 32) following repetitive TMS (rTMS; 3 min, 10 Hz). Main results. This coil could generate a maximum magnetic field of 460 mT and an electric field of 7.2 V m−1 in the rat brain according to our simulations. With subthreshold rTMS focally delivered over the sensorimotor cortex, mean firing rates of primary somatosensory and motor cortical neurons significantly increased; MEP and SSEP amplitude significantly increased, respectively. Significance. This miniaturized C-shaped coil enabled focal TMS and concurrent electrophysiological recording/stimulation at the TMS focal point. It provided a useful tool to investigate the neural responses and underlying mechanisms of TMS in small animal models. Using this paradigm, we for the first time observed distinct modulatory effects on SUAs, SSEPs, and MEPs with the same rTMS protocol in anesthetized rats. These results suggested that multiple neurobiological mechanisms in the sensorimotor pathways were differentially modulated by rTMS.
Klein, E., Daza, N. M., Dasgupta, I., MacDuffie, K., Schönau, A., Flynn, G., Song, D., and Goering, S. Views of stakeholders at risk for dementia about deep brain stimulation for cognition. Brain Stimulation, 2023, DOI: 10.1016/j.brs.2023.04.007.
Dementia at-risk population cautiously supports DBS research for cognition and behavior. Thresholds for considering a future cognitive or behavioral treatment device vary. Hypothetical cognitive and behavioral devices promise benefits and raise ethical concerns.
Thielen, B., Xu, H., Fujii, T., Rangwala, S., Jiang, W., Lin, M., Kammen, A., Liu, C.Y., Selvan, P., Song, D., Mack, W.J., and Meng, E. Making a case for endovascular approaches for neural recording and stimulation. Journal of Neural Engineering, 2023, DOI: 10.1088/1741-2552/acb086.
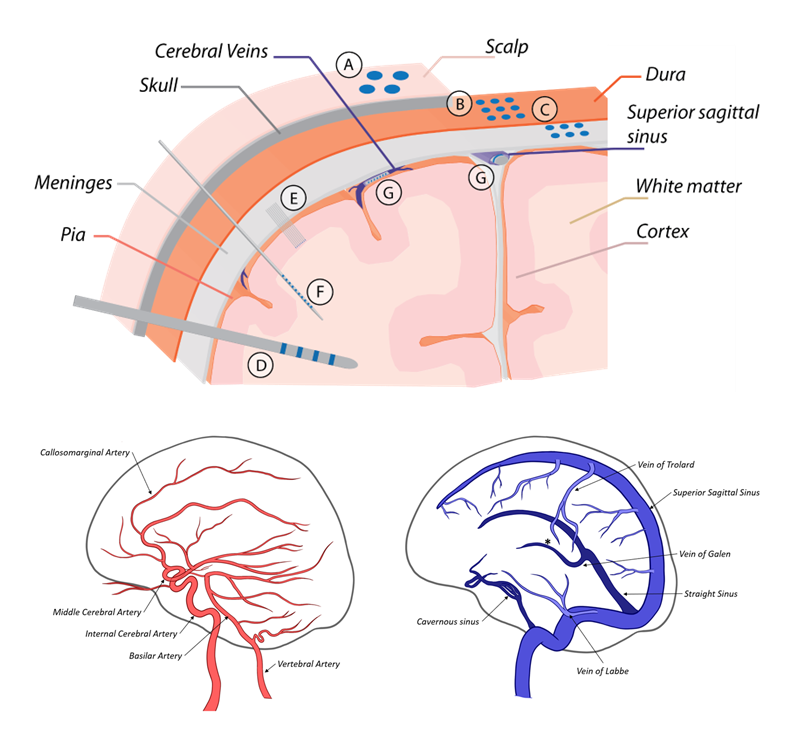
There are many electrode types for recording and stimulating neural tissue, most of which necessitate direct contact with the target tissue. These electrodes range from large, scalp electrodes which are used to non-invasively record averaged, low frequency electrical signals from large areas/volumes of the brain, to penetrating microelectrodes which are implanted directly into neural tissue and interface with one or a few neurons. With the exception of scalp electrodes (which provide very low-resolution recordings), each of these electrodes requires a highly invasive, open brain surgical procedure for implantation, which is accompanied by significant risk to the patient. To mitigate this risk, a minimally invasive endovascular approach can be used. Several types of endovascular electrodes have been developed to be delivered into the blood vessels in the brain via a standard catheterization procedure. In this review, the existing body of research on the development and application of endovascular electrodes is presented. The capabilities of each of these endovascular electrodes is compared to commonly used direct-contact electrodes to demonstrate the relative efficacy of the devices. Potential clinical applications of endovascular recording and stimulation and the advantages of endovascular versus direct-contact approaches are presented.
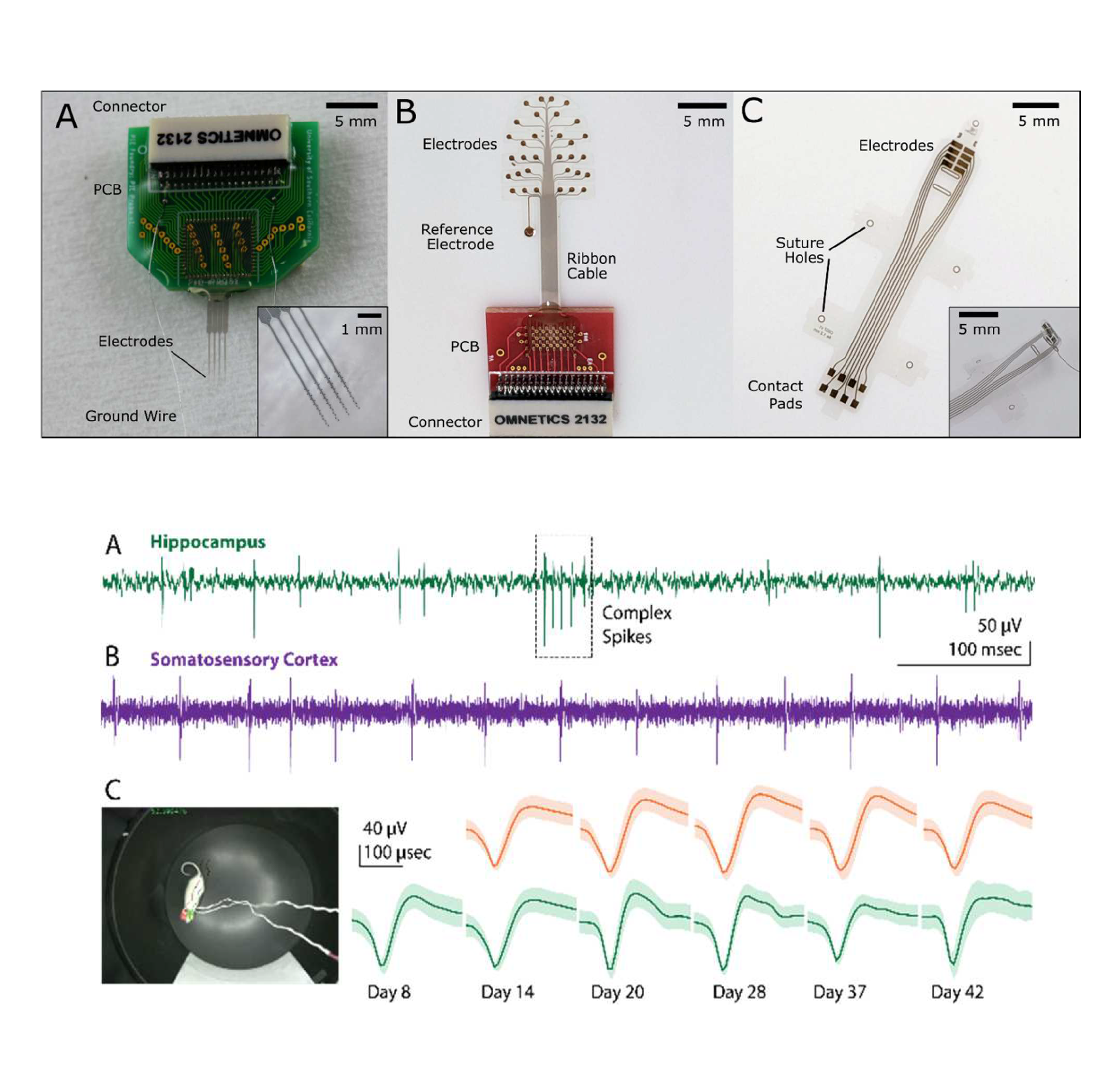
Scholten, K., Xu, H., Song, D., and Meng, E. A shared resource for building polymer-based microelectrode arrays as neural interfaces. Proceedings of the 11th IEEE EMBC Conference on Neural Engineering (NER’23), 2023, DOI: 10.1109/NER52421.2023.10123883.
Chronic functionality of neural interfaces (NI) is hampered by the physiological response to foreign objects, in part due to the mismatch of mechanical properties between soft neural tissue and the rigid materials used in interface construction. Polymer-based NIs have emerged as a key new technology in the pursuit of chronically stable neural recording and stimulation, but most polymer NIs are bespoke devices developed as part of specific research missions; many researchers do not have access to polymer-based NIs technology and among those who do there is a severe lack of standardization in material, construction, packaging, and testing, leading to a lack of repeatability among datasets. Here we present the Polymer Implantable Electrode (PIE) Foundry, a shared-resource for fabricating and disseminating standardized polymer-based microelectrode arrays for use in NIs. The model is based on the successful shared prototyping concept developed for the field of semiconductor research. Professional staff, supported by the BRAIN Initiative funding and operating in cleanroom space provided by the University of Southern California, offer design, fabrication, packaging, and testing of polymer-based microelectrode arrays as a free service to academic and non-profit research groups. The core enabling technology is a standardized set of micromachining protocols applied to the biocompatible, thin-film polymer Parylene C. By leveraging this method, we produce microelectrode arrays of varied size, shape, channel count, and application, disseminating hundreds of arrays to 18+ research groups in our first three years of operation. By standardizing materials, fabrication, and packaging, we create repeatable and comparable devices and have built a library of shareable designs. Channel counts range from 2 to 64, electrode sizes range from 15 μm diameter to 1 mm, designs include penetrating neural probes, spinal paddle electrodes, surface arrays for electroencephalography, and peripheral nerve cuffs for recording and stimulation, animal models include songbird, mouse, rat, cat, and sheep. Here we present details of our organizational structure, fabrication and packaging methods, representative examples of ex vivo and in vivo electrode performance, and key results from the first three years of Foundry operation.
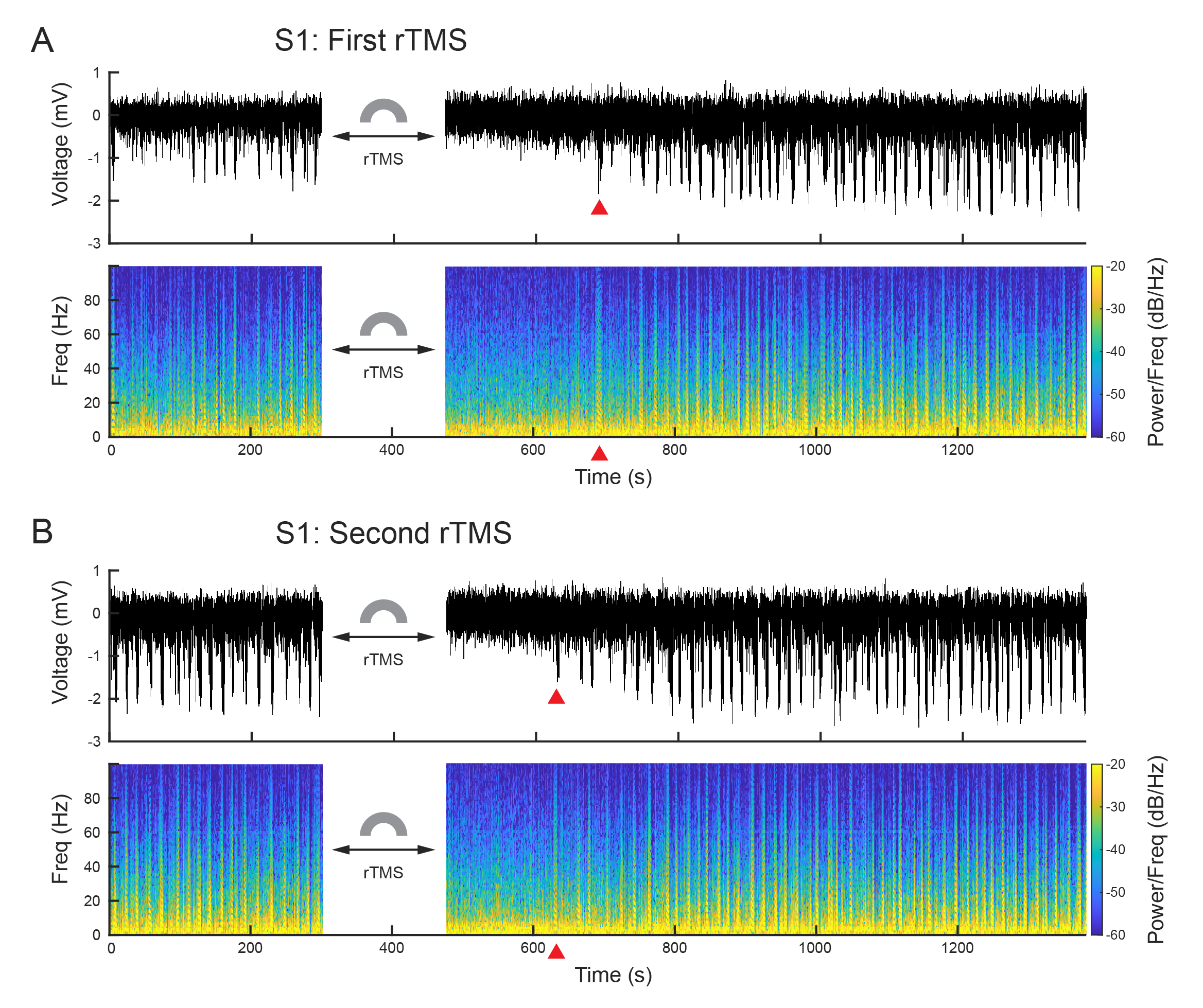
Jiang, W., Isenhart, R., Sutherland, R., Lu, Z., Xu, H., Pace, J., Bonaguidi, M.A., Lee, D., Liu, C.Y., and Song, D. Subthreshold rTMS Suppresses Ketamine-Induced Poly Population Spikes in Rat Sensorimotor Cortex. Frontiers in Neuroscience, 2022, DOI: 10.3389/fnins.2022.998704.
Cortical oscillations within or across brain regions play fundamental roles in sensory, motor, and memory functions. It can be altered by neuromodulations such as repetitive transcranial magnetic stimulation (rTMS) and pharmacological manipulations such as ketamine. However, the neurobiological basis of the effects of rTMS and ketamine, as well as their interactions, on cortical oscillations is not understood. In this study, we developed and applied a rodent model that enabled simultaneous rTMS treatment, pharmacological manipulations, and invasive electrophysiological recordings, which is difficult in humans. Specifically, a miniaturized C-shaped coil was designed and fabricated to deliver focal subthreshold rTMS above the primary somatosensory (S1) and motor (M1) cortex in rats. Multi-electrode arrays were implanted to record local field potentials (LFPs) and single unit activities. A novel form of synchronized activities, poly population spikes (PPS), was discovered as the biomarker of ketamine in LFPs. Brief subthreshold rTMS effectively and reversibly suppressed PPS while increasing the firing rates of single unit activities. These results suggest that ketamine and rTMS have convergent but opposing effects on cortical oscillations and circuits. This highly robust phenomenon has important implications to understanding the neurobiological mechanisms of rTMS and ketamine as well as developing new therapeutic strategies involving both neuromodulation and pharmacological agents.
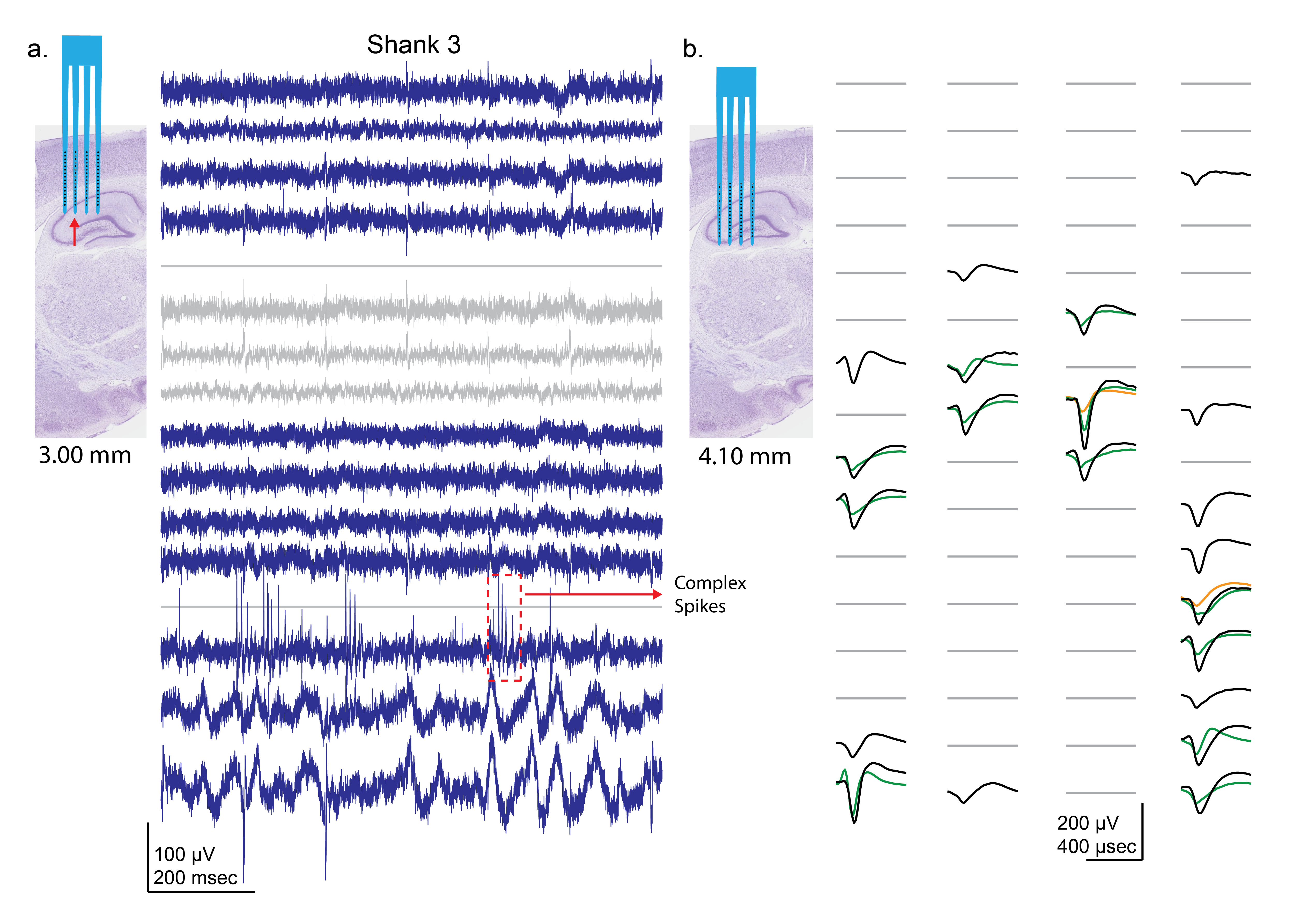
Xu, H., Scholten, K., Jiang, W., Ortigoza-Diaz, J-L., Lu, Z., Liu, X., Meng, E., and Song, D. Generic Parylene Microelectrode Array Implanted with Dip-coating Method into the Rat Brain. Proceedings of the IEEE EMBC Conference, 2022, 224-227.
Flexible polymer-based microelectrode arrays (MEAs) can reduce tissue inflammation and foreign body response and greatly prolong the lifetime of neural implants. However, standard and customized polymer devices are only accessible to limited groups. To better promote the development and application of polymer MEAs, we have launched the Polymer Implantable Electrode (PIE) Foundry and developed a 64-channel Parylene C-based MEA with generic electrodes layout that can be used to record from both cortical and sub-cortical regions in rodents. In addition, a practical dip-coating protocol for the insertion of the flexible standard Parylene MEA is developed.
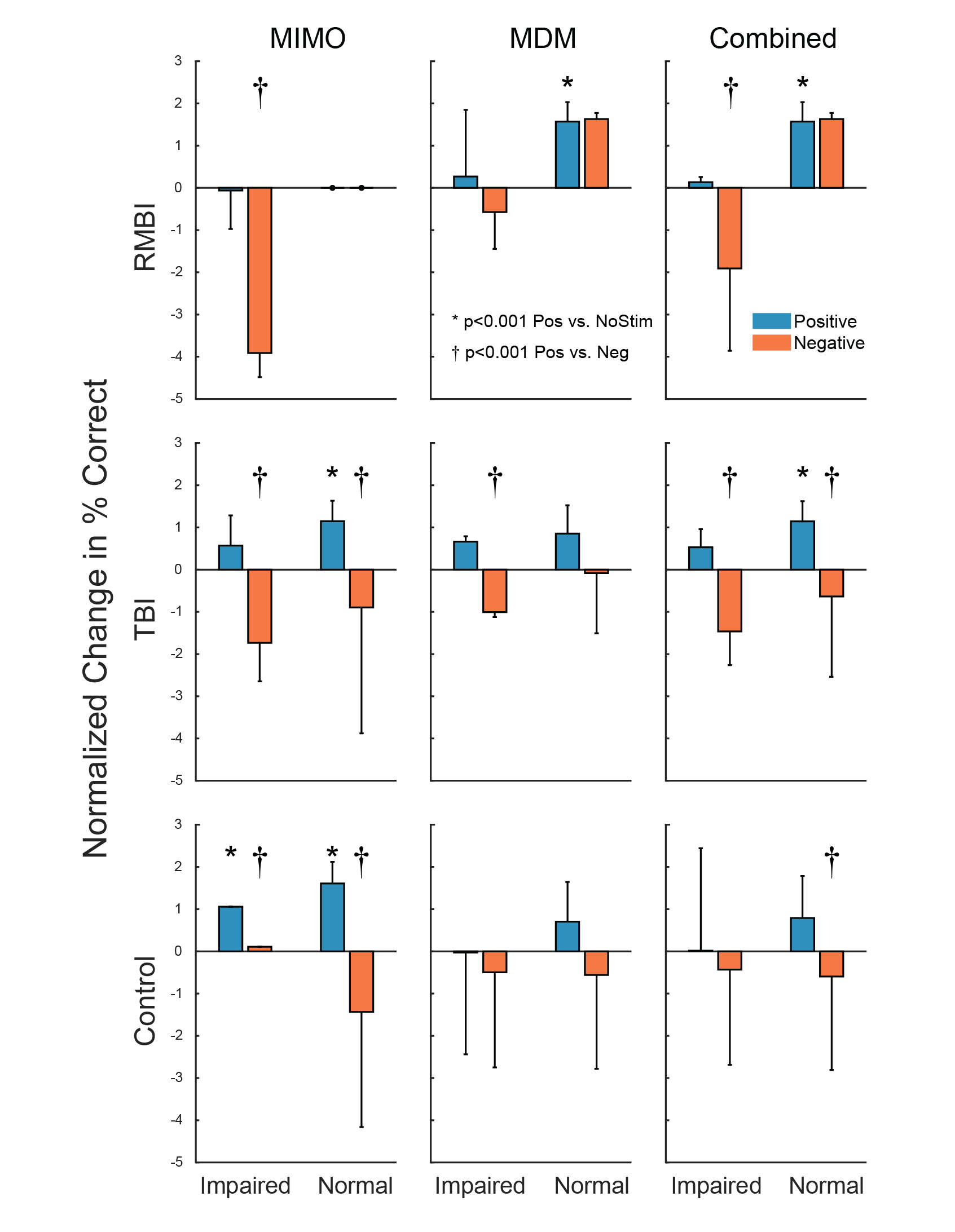
Roeder, B.M., Riley, M.R., She, X., Dakos, A.S., Robinson, B.S., Moore, B.J., Couture, D.E., Laxton, A., Popli, G., Clary, H.M., Sam, M., Heck, C., Nune, G., Lee, B., Liu, C., Shaw, S., Gong, H., Marmarelis, V.Z., Berger, T.W., Deadwyler, S.A., Song, D., Hampson, R.E. Patterned hippocampal stimulation facilitates memory in patients with a history of head impact and/or brain injury. Frontiers in Human Neuroscience, 2022, DOI: 10.3389/fnhum.2022.933401.
Rationale: If deep brain stimulation (DBS) is to be used as a treatment or a prosthetic for memory, it is essential to address various conditions under which different patterns of stimulation may, or may not, be effective. Many pre-clinical DBS paradigms are addressed in epilepsy patients undergoing intracranial monitoring for seizure localization, since they already have electrodes implanted in brain areas of interest. Even though epilepsy is usually not the disorder targeted by DBS, the studies can nevertheless model other memory-impacting disorders, such as Traumatic Brain Injury.
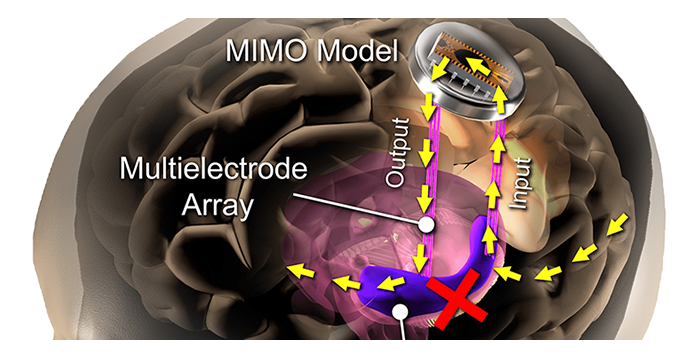
Methods: Human patients undergoing Phase II invasive monitoring for intractable epilepsy, received hippocampal DBS electrodes capable of recording single neurons, ensembles, and EEG signals. On the second day after implant, subjects performed a delayed-match-to-sample (DMS) memory task while hippocampal ensembles from CA1 and CA3 cell layers were recorded. The ensemble activity was correlated to DMS trial events and stimulus (image) content to compute two nonlinear models: a multi-input, multi-output (MIMO) model of CA3-to-CA1 neural encoding, and a Memory Decoding Model (MDM), also based on CA3 and CA1 ensemble activity. Prior to explant (typically 8-14 days after implant), subjects again performed the DMS task while either MIMO-based or MDM-based patterned stimulation was delivered to CA1 electrode sites during the encoding phase of the DMS trials. Memory retention of stimulated vs. non-stimulated trials was assessed using a Delayed Recognition task. Each subject was scored for percentage successful recall on stimulated vs nonstimulated DMS trials. In addition, subjects were sorted (post-hoc) by prior experience of repeated and/or mild-to-moderate brain injury (RMBI), traumatic brain injury (TBI), or no history (control). The subject’s medical history was unknown to the experimenters until after individual subject memory retention results were scored.
Results: When examined compared to control subjects, both TBI and RMBI subjects showed increased memory retention in response to both MIMO and MDM-based hippocampal stimulation. Furthermore, effects of stimulation were also greater than control for subjects who were scored as mild-to-moderate memory impaired in pre-surgical neuropsychological assessment.
Conclusion: These results show that hippocampal stimulation for memory facilitation was more beneficial for subjects who had previously suffered a brain injury (other than epilepsy), compared to control (epilepsy) subjects who had not suffered a brain injury. This study demonstrates that the epilepsy/intracranial recording model can be extended to test the ability of DBS to restore memory function in subjects who previously suffered a brain injury other than epilepsy, and support further investigation into the beneficial effect of DBS in TBI patients.
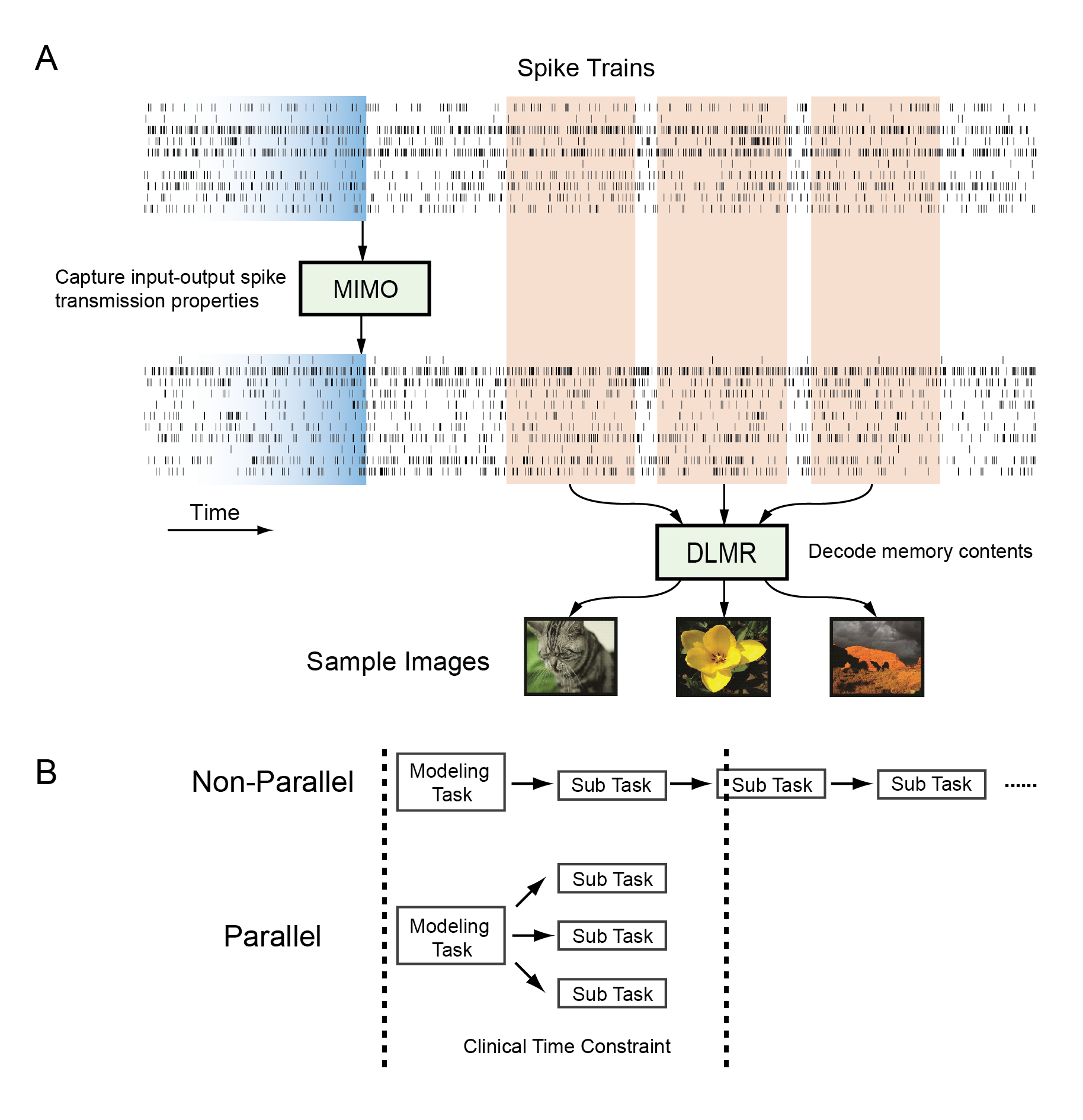
She, X., Robinson, B.S., Flynn, G., Berger, T.W., and Song, D. Accelerating input-output model estimations with parallel computing for testing hippocampal memory prostheses in human. Journal of Neuroscience Methods, 2022, DOI: 10.1016/j.jneumeth.2022.109492.
Hippocampal memory prosthesis is defined as a closed-loop biomimetic system that can be used for restoration and enhancement of memory functions impaired in diseases or injuries. To build such a prosthesis, we have developed two types of input-output models, i.e., a multi-input multi-output (MIMO) model for predicting output spike trains based on input spikes, and a double-layer multi-resolution memory decoding (MD) model for classifying spatio-temporal patterns of spikes into memory categories. Both models can achieve high prediction accuracy using human hippocampal spikes data and can be used to derive electrical stimulation patterns to test the hippocampal memory prosthesis.
However, testing hippocampal memory prostheses in human epilepsy patients with such models has to be performed within a much shorter time window (48–72 h) due to clinical limitations. To solve this problem, we have developed parallelization strategies to decompose the overall model estimation task into multiple independent sub-tasks involving different outputs and cross-validation folds. These sub-tasks are then accomplished in parallel on different computer nodes to reduce model estimation time. Such strategies allow us to complete the modeling procedure within the required time frame to further test input-output model-driven electrical stimulations for the hippocampal memory prosthesis. It has important implications to test the model-based DBS intraoperatively and developing clinically viable hippocampal memory prostheses.
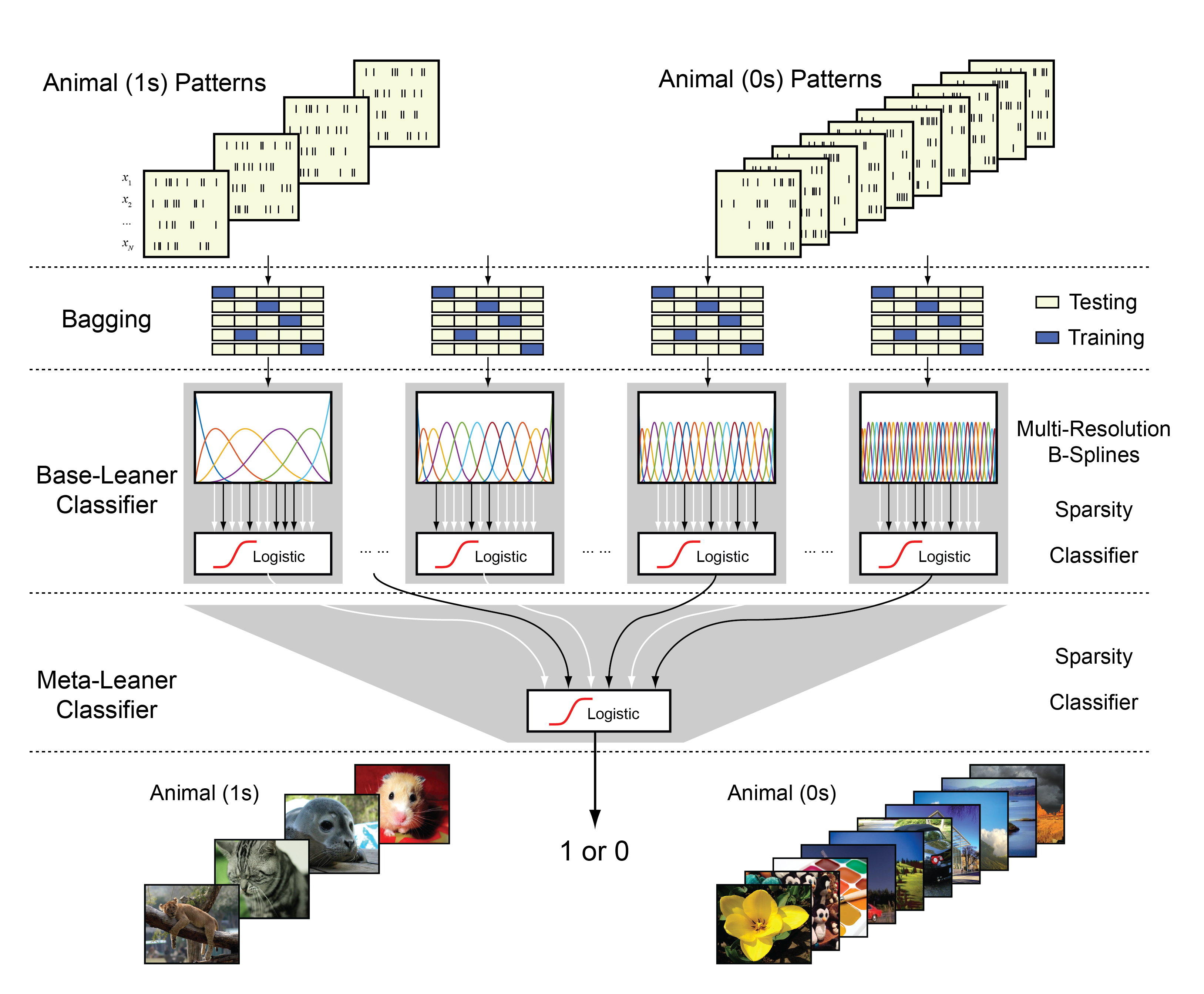
She, X., Berger, T.W., and Song, D. A double-layer multi-resolution classification model for decoding spatio-temporal patterns of spikes. Neural Computation, 2021, DOI: 10.1162/neco_a_01459.
We build a double-layer, multiple temporal-resolution classification model for decoding single-trial spatiotemporal patterns of spikes. The model takes spiking activities as input signals and binary behavioral or cognitive variables as output signals and represents the input-output mapping with a double-layer ensemble classifier. In the first layer, to solve the underdetermined problem caused by the small sample size and the very high dimensionality of input signals, B-spline functional expansion and L1-regularized logistic classifiers are used to reduce dimensionality and yield sparse model estimations. A wide range of temporal resolutions of neural features is included by using a large number of classifiers with different numbers of B-spline knots. Each classifier serves as a base learner to classify spatiotemporal patterns into the probability of the output label with a single temporal resolution. A bootstrap aggregating strategy is used to reduce the estimation variances of these classifiers. In the second layer, another L1-regularized logistic classifier takes outputs of first-layer classifiers as inputs to generate the final output predictions. This classifier serves as a meta-learner that fuses multiple temporal resolutions to classify spatiotemporal patterns of spikes into binary output labels. We test this decoding model with both synthetic and experimental data recorded from rats and human subjects performing memory-dependent behavioral tasks. Results show that this method can effectively avoid overfitting and yield accurate prediction of output labels with small sample size. The double-layer, multi-resolution classifier consistently outperforms the best single-layer, single-resolution classifier by extracting and utilizing multi-resolution spatiotemporal features of spike patterns in the classification.
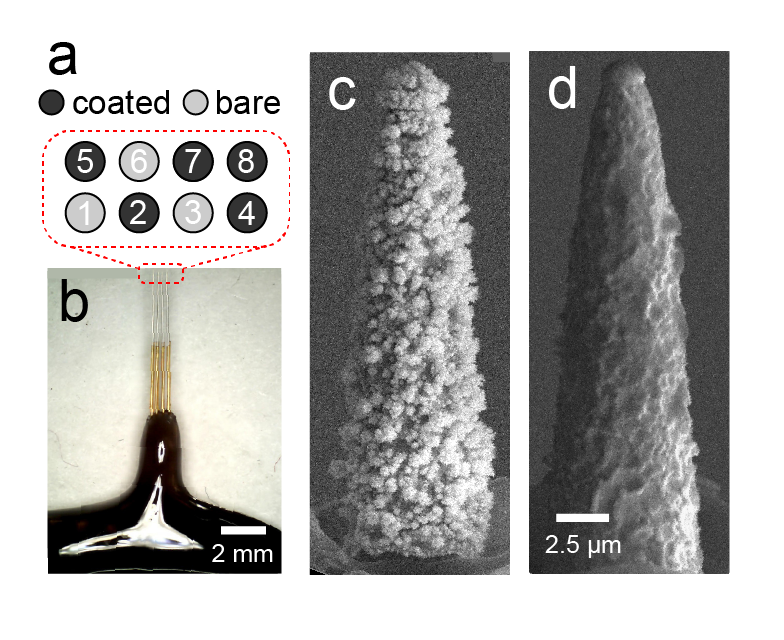
Elyahoodayan, S., Jiang, W., Lee, C., Shao, A., Weiland, G., Whalen, J., Petrossians, A., and Song, D. Stimulation of recording of the hippocampus using the same Pt-Ir Coated Microelectrodes. Frontiers in Neuroscience, 2021, DOI: 10.3389/fnins.2021.616063.
Same-electrode stimulation and recording with high spatial resolution, signal quality, and power efficiency is highly desirable in neuroscience and neural engineering. High spatial resolution and signal-to-noise ratio is necessary for obtaining unitary activities and delivering focal stimulations. Power efficiency is critical for battery-operated implantable neural interfaces. This study demonstrates the capability of recording single units as well as evoked potentials in response to a wide range of electrochemically safe stimulation pulses through high-resolution microelectrodes coated with co-deposition of Pt-Ir. It also compares signal-to-noise ratio, single unit activity, and power efficiencies between Pt-Ir coated and uncoated microelectrodes. To enable stimulation and recording with the same microelectrodes, microelectrode arrays were treated with electrodeposited platinum-iridium coating (EPIC) and tested in the CA1 cell body layer of rat hippocampi. The electrodes’ ability to (1) inject a large range of electrochemically reversable stimulation pulses to the tissue, and (2) record evoked potentials and single unit activities were quantitively assessed over an acute time period. Compared to uncoated electrodes, EPIC electrodes recorded signals with higher signal-to-noise ratios (coated: 9.77 ± 1.95 dB; uncoated: 1.95 ± 0.40 dB) and generated lower voltages (coated: 100 mV; uncoated: 650 mV) for a given stimulus (5 μA). The improved performance corresponded to lower energy consumptions and electrochemically safe stimulation above 5 μA (>0.38 mC/cm2), which enabled elicitation of field excitatory post synaptic potentials and population spikes. Spontaneous single unit activities were also modulated by varying stimulation intensities and monitored through the same electrodes. This work represents an example of stimulation and recording single unit activities from the same microelectrode, which provides a powerful tool for monitoring and manipulating neural circuits at the single neuron level.
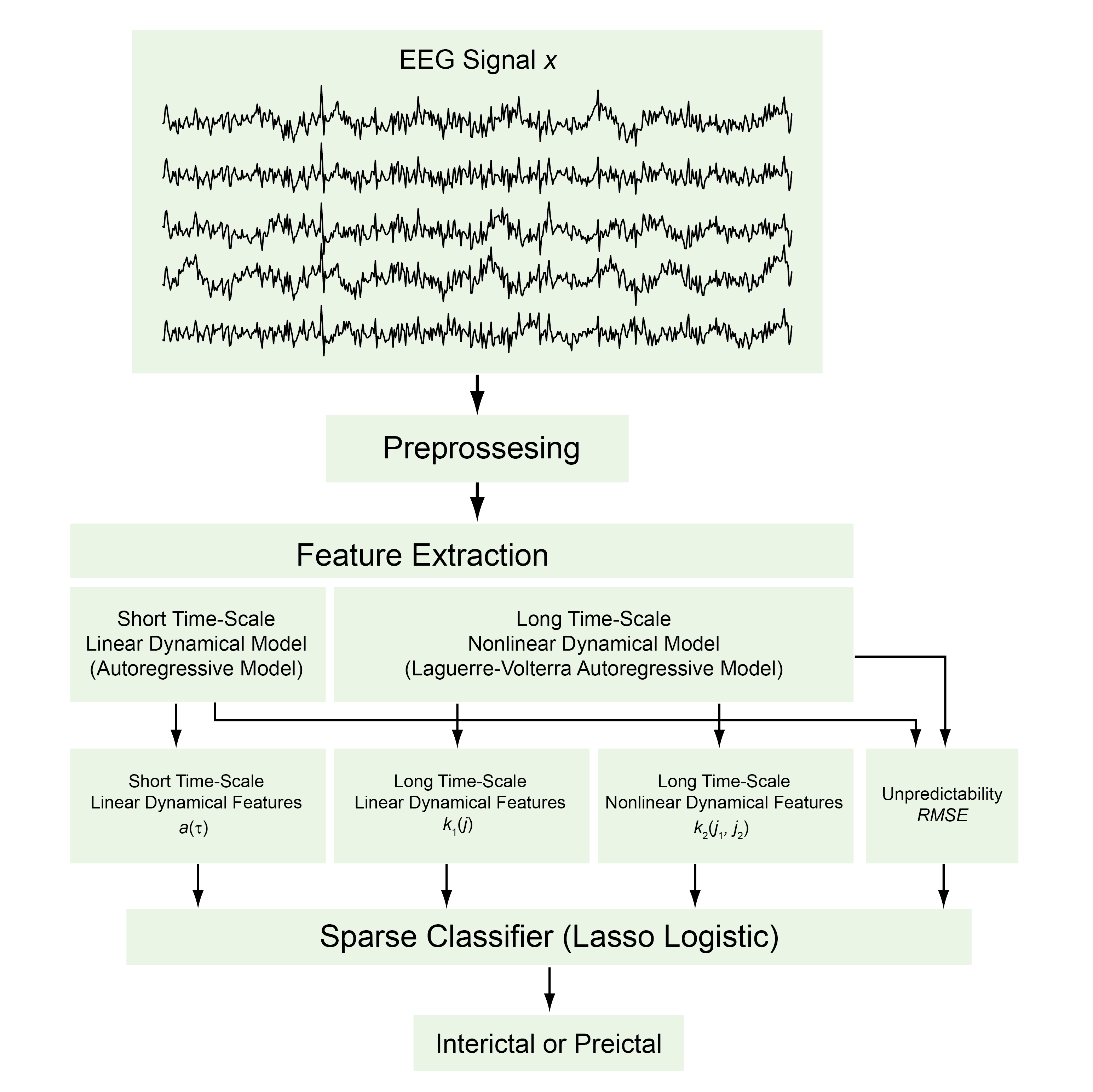
Yu, P-N., Liu, C.Y., Heck, C.N., Berger, T.W. and Song, D. A Sparse multiscale nonlinear autoregressive model for seizure prediction. Journal of Neural Engineering, 2021, DOI: 10.1088/1741-2552/abdd43.
Accurate seizure prediction is highly desirable for medical interventions such as responsive electrical stimulation. We aim to develop a classification model that can predict seizures by identifying preictal states, i.e. the precursor of a seizure, based on multi-channel intracranial electroencephalography (iEEG) signals. Approach. A two-level sparse multiscale classification model was developed to classify interictal and preictal states from iEEG data. In the first level, short time-scale linear dynamical features were extracted as autoregressive (AR) model coefficients; arbitrary (usually long) time-scale linear and nonlinear dynamical features were extracted as Laguerre–Volterra AR model coefficients; root-mean-square error of model prediction was used as a feature representing model unpredictability. In the second level, all features were fed into a sparse classifier to discriminate the iEEG data between interictal and preictal states. Main results. The two-level model can accurately classify seizure states using iEEG data recorded from ten canine and human subjects. Adding arbitrary (usually long) time-scale and nonlinear features significantly improves model performance compared with the conventional AR modeling approach. There is a high degree of variability in the types of features contributing to seizure prediction across different subjects. Significance. This study suggests that seizure generation may involve distinct linear/nonlinear dynamical processes caused by different underlying neurobiological mechanisms. It is necessary to build patient-specific classification models with a wide range of dynamical features.
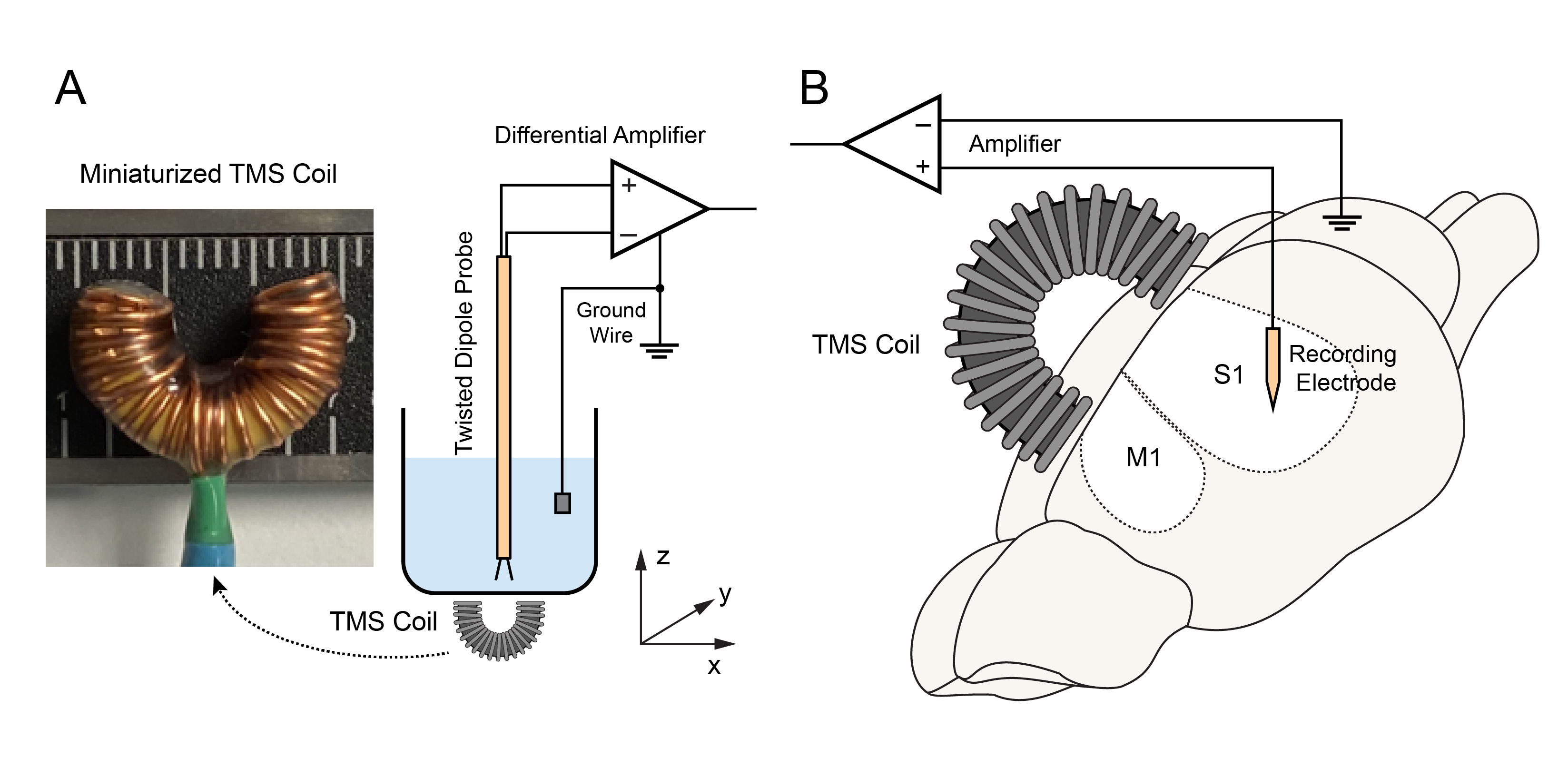
Jiang, W., Isenhart, R., Kistler, N., Lu, Z., Xu, H., Lee, D., Liu, C.Y., and Song, D. Low intensity repetitive transcranial magnetic stimulation modulates spontaneous spiking activities in rat cortex. Proceedings of the IEEE EMBC Conference, 2021, 6318-6321.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique for neuromodulation. Even at low intensities, rTMS can alter the structure and function of neural circuits; yet the underlying mechanism remains unclear. Here we report a new experimental paradigm for studying the effect of low intensity rTMS (LI-rTMS) on single neuron spiking activities in the sensorimotor cortex of anesthetized rats. We designed, built, and tested a miniaturized TMS coil for use on small animals such as rats. The induced electric field in different 3D locations was measured along different directions using a dipole probe. A maximum electric field strength of 2.3 V/m was achieved. LI-rTMS (10 Hz, 3 min) was delivered to the rat primary motor and somatosensory cortices. Single-unit activities were recorded before and after LI-rTMS. Results showed that LI-rTMS increased the spontaneous firing rates of primary motor and somatosensory cortical neurons. Diverse modulatory patterns were observed in different neurons. These results indicated the feasibility of using miniaturized coil in rodents as an experimental platform for evaluating the effect of LI-rTMS on the brain and developing therapeutic strategies for treating neurological disorders.

